Add to Cart
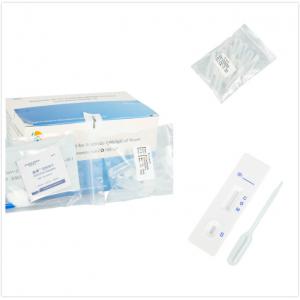
COVID-19 IgG/IgM Rapid Test Quick sampling by fingertip blood, Result in 10 minutes
Product Description:
Limitations of COVID-19 IgM/IgG Rapid Test Kit:
1. COVID-19 Rapid Test is for in vitro diagnostic use only. The test should be performed using serum, plasma or whole blood samples only. Neither the quantitative value nor the rate of increase in COVID-19 antibody concentration can be determined by this qualitative test.
2. In the early onset of fever, anti-COVID-19 IgM concentrations may be below detectable levels.
3. The continued presence or absence of antibodies cannot be used to determine the success or failure of therapy.
4. Results from immunosuppressed patients should be interpreted with caution.
5. As with all diagnostic tests, all results must be interpreted together with other clinical information available to the physician.
6. If the test result is negative and clinical symptoms persist additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of COVID-19 infection.
Product Feasure:
| Product Name | COVID-19 IgG/IgM Rapid Test Quick sampling by fingertip blood, Result in 10 minutes |
| Sample type | serum, plasma, venous whole blood |
| Packing Specification | 10 Test Sets /Kit; 20 Test Sets /Kit |
| Storage Condition | 2℃~30℃ |
| Interpretation of Test Result | 15 minutes to interpret the result |
| Detection mode | single detection, separate detection of IgM and IgG, the two results are non-interference and can complement each other |
| China Registration | Registration certificate No.(National Medical Products Administration approval No.20203400240) |
| International Registration | CE mark |
| Application | It is mainly used in the detection of influenza virus. |
Product Show
How Does the Coronavirus Rapid Test Kit Work?
